The report will include the approvals of quasi-drugs from regulatory organizations, market research, and the competitive aspect of Quasi-drugs. Special emphasis is given to the regulatory approvals in Japan and other countries in Asia pacific regions.
Under the Pharmaceutical and Medical Device Act (PMD Act), certain products are categorized as either cosmetics or quasi-drugs in Japan. Quasi-drugs are products that are somewhere in between cosmetics and pharmaceuticals. It has been defined as “Products with mild action on the human body, they are considered as cosmetics with mild pharmacological action.”
Quasi-drug categories have been specified in Announcement No. 25 of the Ministry of Health from February 6, 2009, and there are currently 27 quasi-drug categories.
Classification of Quasi-Drugs
Quasi Drugs can be classified into 3 categories. Group 1 has items used for sanitary purposes such as sanitary pads, tampons, and menstrual pads. The textile used for manufacturing masks such as dust and surgical masks. Group 2 includes Odor inhibitors like toothpaste, antiperspirants, and bath products. Haircare products that are only meant for external usage. Group 3 has products used for disease prevention and Disinfectants not directly applied to humans.
Purpose of Quasi-Drugs
Prevention of nausea or another discomfort, foul breath, or body odor. Prevention of prickly heat, sores, and the like.Prevention of hair loss, to promote hair growth, or for hair removal. Eradication of or repellence of rats, flies, mosquitoes, fleas, etc. for the health of man or other animals.
To know about digitalization in the beauty industry, click here.
Quasi-Drugs Products Ranking
The graphical representation presents the amount of production of Quasi-Drugs by the therapeutic category of drugs based on the order of amounts of production in 2018 and 2017. The production in the field of Medicated Cosmetics increased in 2018 as compared to 2017. The global quasi-drugs market is expected to gain market growth in the forecast period of 2020 to 2027.
Quasi-Drugs Market
Asia-Pacific is expected to account for the largest market share over the coming years for the quasi-drugs market due to increased general skin problems, increased awareness for quasi-drugs through advertisement and media, and rapidly improving health care infrastructure in the region.
The Market Scenario of Quasi-drugs is quite promising. The Global quasi-drugs market is expected to gain huge growth in the forecast period of 2020-2027 in the market. Opportunities involve growing demand for cosmetic products that do not need medical prescriptions. The growth drivers include rising awareness of oral and personal hygiene among the population. Also growing skin-related problems like acne, dull skin, and more are responsible.
The restraints are lack of access and awareness of the benefits of using quasi Drugs. The challenges include approvals and large-scale competitions among global and local brands.
Quasi-Drugs Categorization
Specific products may be categorized as cosmetics in one market and as drugs or quasi-drugs in other markets.
Examples of different categorizations of products are illustrated:
| Function/ Claim | Europe | Japan | Korea |
| Hair Dye | Cosmetics | Quasi-drug | General/ functional cosmetics |
| Skin Whitener | Cosmetics/ drug | Quasi-drug | General cosmetics |
| Sunscreen | Cosmetics | Cosmetics/ quasi-drug | Functional cosmetics |
| Hair Growth | Cosmetics/ drug | Quasi-drug | Functional cosmetics |
| Depilatory | Cosmetics | Quasi-drug | Functional cosmetics |
| Body Beauty | Cosmetics/ drug | Depending on a specific case | Drug |
| Deodorant | Cosmetics | Quasi-drug | Quasi-drug |
Medicated white cream TR
It has Tranexamic acid as an active ingredient. In addition to a whitening effect, a female hormone-like action, an increase in the water content of a horny layer, inhibiting. Trans-epidermal water loss, ameliorating rough pores are expected.
Medicated UV white TR
It has Tranexamic acid, StearylGlycyrrhetinate. SPF 50, PA+++ acquired. Paraben-free, Preservative-free, Alcohol-free, Synthetic scent-free.
ATP essence lotion
Water-soluble placenta extract, Dipotassium Glycyrrhizinate as an active ingredient. Moist feel lotion is good for delicate skin with whitening effect and rough dry skin improving effect. Paraben-free, Synthetic scent-free, Coloring agent-free, Petroleum surfactant agent-free, Mineral oil-free
Medicated beauty essence AC
It has Dipotassium Glycyrrhizinate. Beauty essence controls sebum secretion, makes the skin firm and bouncy, and prevents rough dry skin and acne .
Medicated mild remover
It has Thioglycolic acid calcium as an active ingredient. Inhibiting the peculiar smell of depilatory, capable to feel its effect soon, cream type is easy to use. Moisturizing ingredients and plant extract ingredients included.
Medicated hair tonic
Dipotassium Glycyrrhizinate Acetate DL-alpha-tocopherol Assembly extracts as an active ingredient. In addition to the effective ingredients, Japanese and Chinese plant extract ingredients are added. Preservative-free.
Regulation of Quasi Drugs
If you are planning to launch the products in Japan specifically, then you will need to follow the procedure and get approvals from regulatory bodies’ approval.
Quasi-drugs are subject to pre-market approval and licensing requirements. Registration of all ingredients used in product manufacture, as well as product safety data which specify the active ingredients, usage and dosage, indications or effects is also required.
Full lists of approved quasi-drug ingredients are not published, although the MHLW has published lists of ingredients approved for use in certain categories. Full ingredient listing is not required for quasi-drugs; however, the MHLW has listed 138 ingredients that must be indicated on the label. There are prescribed safety tests for quasi-drugs, although the data required for approval vary depending on whether the product is a new quasi-drug or a recognized previously approved quasi-drug.
To get the complete list of regulatory bodies in different regions across the globe, contact Signicent.
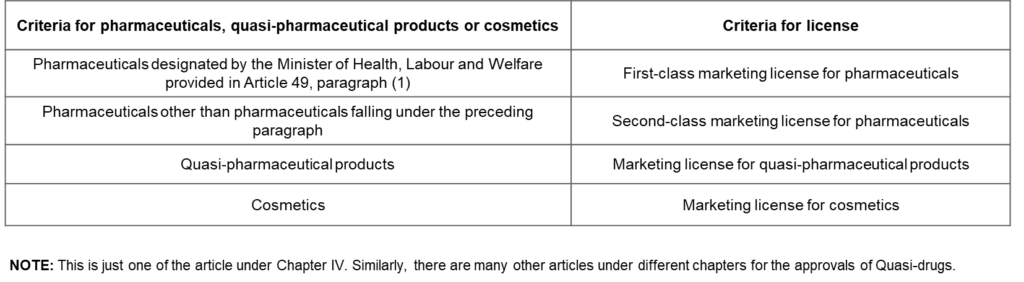
Approval articles for Quasi-Drugs
Marketing and Manufacturing of Pharmaceuticals, Quasi-Pharmaceutical Products and Cosmetics (Articles 12 to 23) Article 12(1). In accordance with the criteria for pharmaceuticals (excluding in-vitro diagnostics; hereinafter the same applies in this Chapter), quasi-pharmaceutical products or cosmetics set forth in the left-hand columns of the following table, no person other than one who has obtained a license from the Minister of Health, Labour and Welfare specified in the right-hand columns of the same table respectively, may engage in the business of marketing pharmaceuticals, quasi-pharmaceutical products or cosmetics.
Regulatory Authorities in APAC
The regulatory authorities in the APAC region include (PMDA) Pharmaceutical and Medical Device Agency, (MHLW) Ministry of Health, Labour and Welfare, (TGA) Therapeutic Goods Administration, (ACCC) Australian Competition and Consumer Commission, (CDSCO) Central Drugs Standard Control Organization, (MFDS) Ministry of Food and Drug Safety.
Applicable regulations for cosmetics in Korea
The overarching type needs Cosmetic Act (No.14027) (2014.07.31), Enforcement Decrees of Cosmetics Act (No. 27827), Enforcement Rules of Cosmetics Act (No. 1357) (Enforcement date, 2017.05.30).
On the basis of ingredients Regulations for Designation of Cosmetic Ingredients(2017-3) ) (Enforcement date, 2017.07.01). Types, Standards, and Test Methods of Cosmetic Color Additives (2016-49).
Labelling Regulations on the Demonstration of Labeling and Advertisement for Cosmetic Products(2014-80). On the basis of Testing the Types and Standards of Cosmetics and Test Methods(2016-49) are concerned with regulations.
For manufacturing, the regulations required are Regulations on Cosmetic Good Manufacturing and Quality Control Practices(2015-58). For Functional Cosmetics Regulations on the Examination of Functional Cosmetics(2015-14) as well as the Standards and Test Methods of Functional Cosmetics(2015-15) are the concerned regulatory bodies.
Each year Signicent provides consultancy to hundreds of organizations to help transform their innovations to value.
Competitive Analysis
Pola Chemical Industries, INC. with its headquarter in Yokohama, Kanagawa, JAPAN has a product, POLA Wrinkle Shot Medical Serum. The Active Ingredients involve Sodium [[[trifluoro-isopropyl-oxopropyl] aminocarbonyl]pyrrolidinyl]carbonyl]-methylpropyl]aminocarbonyl]benzoylamino]acetate. It took 15 years of research to release the product in the market. The product was launched in early 2017. An active ingredient that inhibits the activity of neutrophil elastase, an enzyme that causes wrinkles. Over 5000 substances were investigated. The company is aggressively working in Sustainability.
Serum For Wrinkles
Process of Wrinkle Formation: Mild inflammation of the skin normally occurs due to exposure to stimuli such as ultraviolet rays and dryness from the external environment. It causes deterioration of extracellular matrix components (collagen and elastin). Over the long term this process, together with the creasing of the skin due to facial expressions, simultaneously exacerbate wrinkles.
Improving wrinkles: The inhibitory activity of neutrophil elastase causes the breakdown and should be targeted. Wrinkles Inflammation in the dermis causes neutrophil (a type of lymphocyte) infiltration, leading to excessive secretion of neutrophil elastase. •This enzyme breaks down and reduces the number of extracellular matrix components such as collagen and elastin in the dermis.
They conducted a double-masked, randomized trial comparing a preparation containing the active ingredient (The Serum) with a preparation without the active ingredient (Placebo). The Serum and Placebo were applied twice a day for 12 weeks to the corner of one eye each, in 68 healthy Japanese women with wrinkles at the corners of the eyes.
Visual evaluation & instrumental evaluations were performed in the 12th week of use, which showed significant improvement of wrinkles with the Serum compared to Placebo use.
Collaborations in the Market
Pola Chemicals is collaborating with PeptiDream
Pola Orbis develops, manufactures, and markets cosmetic products, centering in skincare products. Under its multi-brand strategy, the Company holds nine cosmetic brands including POLA, prestige skincare brand, ORBIS, oil-free skincare brand, H2O PLUS, and Jurlique. PeptiDream Inc. is a public biopharmaceutical company founded in 2006 employing our proprietary Peptide Discovery Platform System (PDPS), a state-of-the-art highly versatile discovery platform that enables the production of highly diverse (trillions) non-standard peptide libraries, which then can be developed into peptide-based or small molecule-based therapeutics.
Pola Chemicals & PeptiDream are jointly collaborating for the discovery and development of novel therapeutics, quasi-drugs, and cosmetics that make use of peptides. The combination of the basic research expertise of POLA CHEMICAL INDUSTRIES, INC. in dermatology and PeptiDream’s technology for creating and identifying peptides with novel functions will enable the companies to accelerate R&D for innovations.
Product For Skin
Showa Denko K. K. (Japan) has a product TPNa™ to enhance skin moisturization, Anti-oxidation, and Anti-aging effect. The Active Ingredient is INCI Sodium tocopheryyl phosphate. TPNa™ is registered as an active ingredient for Quasi Drug in Japan for the prevention and cure of rough dry skin and Quasi Drug Additive for anti-oxidant.
TPNa™ which has a phosphoryl group quickly penetrates into the skin and is converted to vitamin E by the enzymatic action of a phosphatase present in the skin. As a result, ROS (Reactive Oxygen Species) can be removed.
